Developmental engineering
Recent research activity
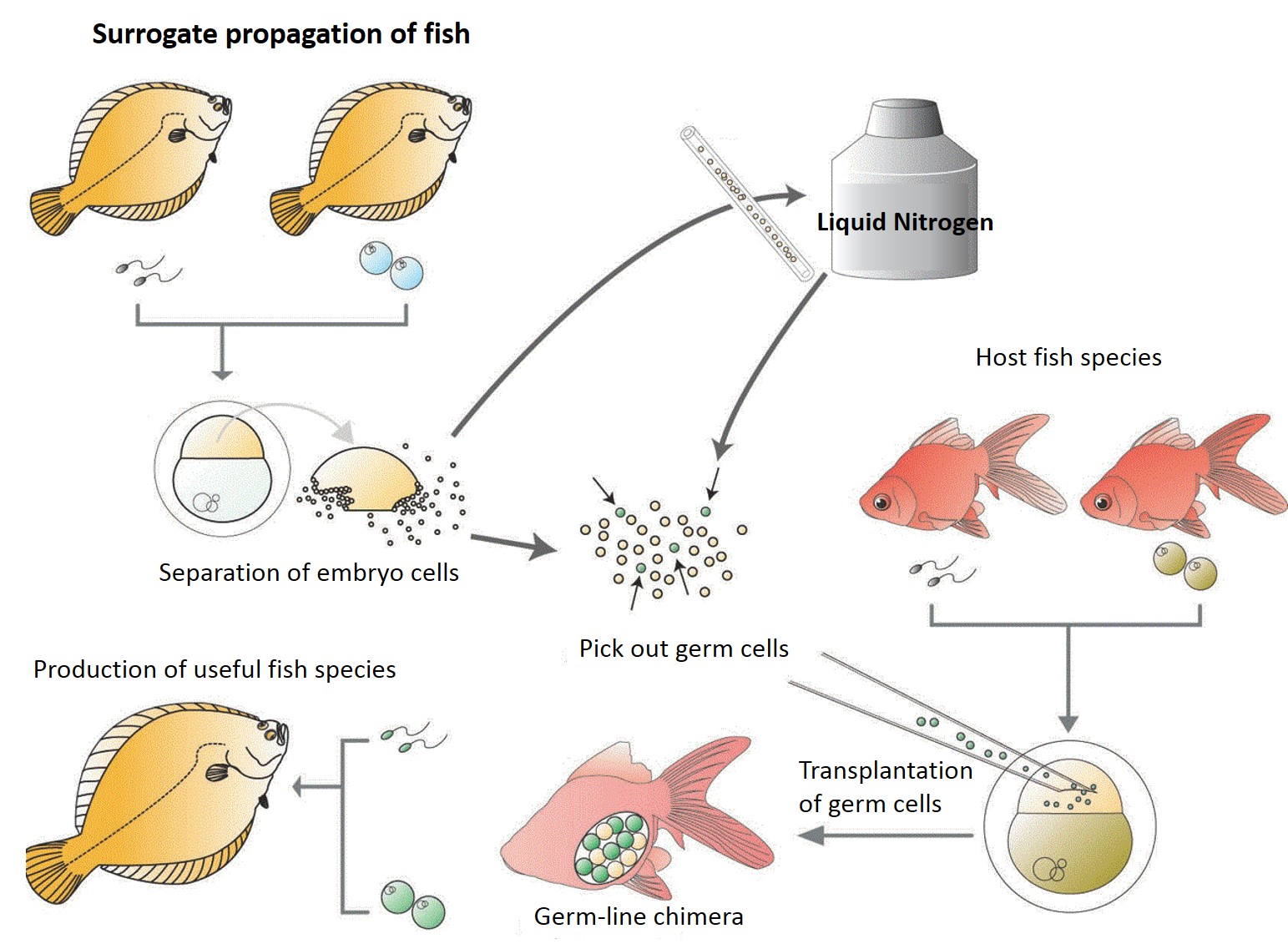
Surrogate
propagation of fish
The term, surrogate, is usually used as “surrogate mother” in reproductive
medicine. Surrogate mother is a mother who bears a child on behalf
of another woman by the implantation in her womb of a fertilized egg from
the other woman. The word surrogate is also used as “surrogate production”
in the field of animal husbandry. For example, fertilized eggs from
beef cattle that have high market value are transplanted into cows to induce
milk production as well as beef cattle baby. Surrogate production
in the aquaculture can also be possible by applying similar technique to
the teleost fish.
Surrogate production in teleost fish is achieved by inducing the germ-line
chimeras. Primordial germ cells (PGCs) are the germ cells before
their arrival at the genital anlage during embryonic stage. To make
germ-line chimeras, the PGCs are isolated and transplanted into the embryo
of other species. The hosts become germ-line chimeras if the transplanted
PGCs migrated to genital anlage and differentiated into functional gametes.
In consequence, donor genotypes are restored into the next generation.
The productivity of fish seed is expected to increase by surrogate production
using germ-line chimeras by two different species whose biological properties
are different each other. For example, life cycle of the fish could
be extremely shortened if the surrogate parents that have shorter life
cycle are produced.
Germ-line chimeras in teleost fish are induced by the following methods:
(1) blastomere transplantation, (2) transplantation of a blastoderm graft,
and (3) transplantation of isolated PGCs. In blastomere transplantation,
blastomeres at the blastula stage are randomly sucked by glass micro-needle,
and transplanted into the embryo of other species at the same stage.
In this method, donor cells do not always contain PGCs due to the random
separation of cells from donor blastoderm. In the transplantation
of a blastoderm graft, lower part of the blastula blastoderm, in which
PGCs are contained, is transplanted into the host embryo. In this
method, germ-line chimeras can be always induced. In both cases,
however, the donor cells do not always contribute the development of resultant
chimeric embryos, because selective aggregation of donor cells frequently
occurs.
More effective method is the transplantation of isolated early germ cells,
such as oogonia and spermatogonia. In salmonids, germ-line chimeras
are successfully induced, when the germ cells at early stage isolated from
the ovary and testis are transplanted into the abdominal cavity of the
hatched embryos. In this case, functional gametes developed from
donor germ cells were obtained from the host parents.
In
this Station, surrogate production has been studied by using PGCs from many
kinds of teleost fish species.
Procedures
inducing germ-line chimera
Germ-line is induced by transplantation of early stage germ cells into
host embryos. These germ cells are required to maintain motile activity
to host gonad and developmental potency to gametes. Therefore, PGCs
and gonia are appropriate as donor cells to induce germ-line chimera.
Isolated PGCs and germ cells are transplanted into host embryo by using
micro-glass needle under the stereomicroscope. The size of PGCs is
about 10 to 20 mm in diameter. The position in blastoderm where PGCs
are transplanted is important factor for successful migration of PGCs to
host gonad. Therefore, the transplantation is important, very skillful
and laborious work. PGCs from different kinds of teleost species,
such as eel, flatfish, and sturgeon, have migration activity to the gonad
of zebrafish or goldfish.
PGCs migrated successfully to host gonad do not always differentiate to
germ cells, because of intervention or non-support between donor germ cell
and host somatic cells. Functional gametes are induced in interspecific
donor and host combinations, while only functional sperm in interfamilial.
It is difficult to differentiation functional sperm in inter-order transplantation,
because of extinction of donor PGCs during subsequent development.
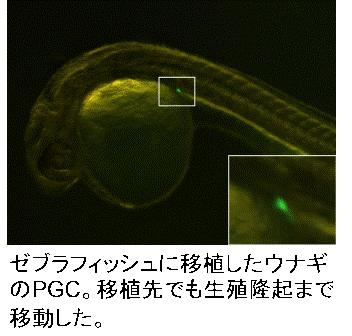
Visualization and
isolation of teleost PGCs
PGCs differentiate at early stage of development in teleost fish.
As difficulty to distinguish them from other somatic cells in early embryo,
visualization of PGCs is required for their isolation and implantation.
Injection of the artificial mRNA, in which GFP sequence is conjugated
with zebrafish nos1 3’ UTR to the fertilized eggs can induce PGC visualization
after the somitogenesis stage. We have succeeded to visualize PGCs
in 17 fish species, such as herring, loach and ice goby, belonging to 6
order, using this technique. Visualized PGCs are able to isolate
from somatic cells by using cell-sorter.
When the visualized PGCs were isolated and transplanted into hetero-chronous
host blastula, donor PGCs migrated to genital anlage in the chimeras between
loach and goldfish (inter-familial transplantation). However, more
detailed studies on molecular mechanisms of PGCs differentiation and migration
is required to introduce the donor PGCs into xenogeneic host genital anlage.
Functional gametes from donor PGCs can be intra-specifically obtained through
germ-line chimeras in many teleost. Functional eggs were also obtained
from inter-subspecific chimeras induced by graft transplantation of blastoderm
between diploid goldfish and triploid crucian carp. Sperm production
in the inter-specific chimeric fish between donor masu salmon and host
rainbow trout was also reported.
Cryopreservation and
vitrification of PGCs
Today genetic diversity decreases rapidly. It is important to preserve
PGCs. As PGCs contain genetic factors in cytoplasm, diversity of
mitochondrial DNAs is also preserved. PGCs preservation means that
of genetic diversity. Genetic diversity also decreases during pedigree
preservation necessarily. Genetic properties in existence will be
useful order to improve genetically future strains and to induce through
germ-line chimera whenever.

Collection of genetic
diversity from natural population
If PGCs are isolated from larva, we may be able to collect large genetic
diversity from natural population in spawning area. Many marine species
spawn large number of eggs around spawning area, but almost all offspring
die during early stage of development. Therefore, collection of PGCs
around this stage does not damage the natural genetic resource. Moreover,
genetic diversity of this stage is larger than that of individuals at later
stages. Individuals that are not at all afraid of men, are very useful
for aquaculture, but are difficult to survive in natural condition.
But it is not performed yet to isolate PGCs from larva collected offshore.
It is important to elucidate basic biological properties of PGCs,
because of establishment of the new isolation technique of them.
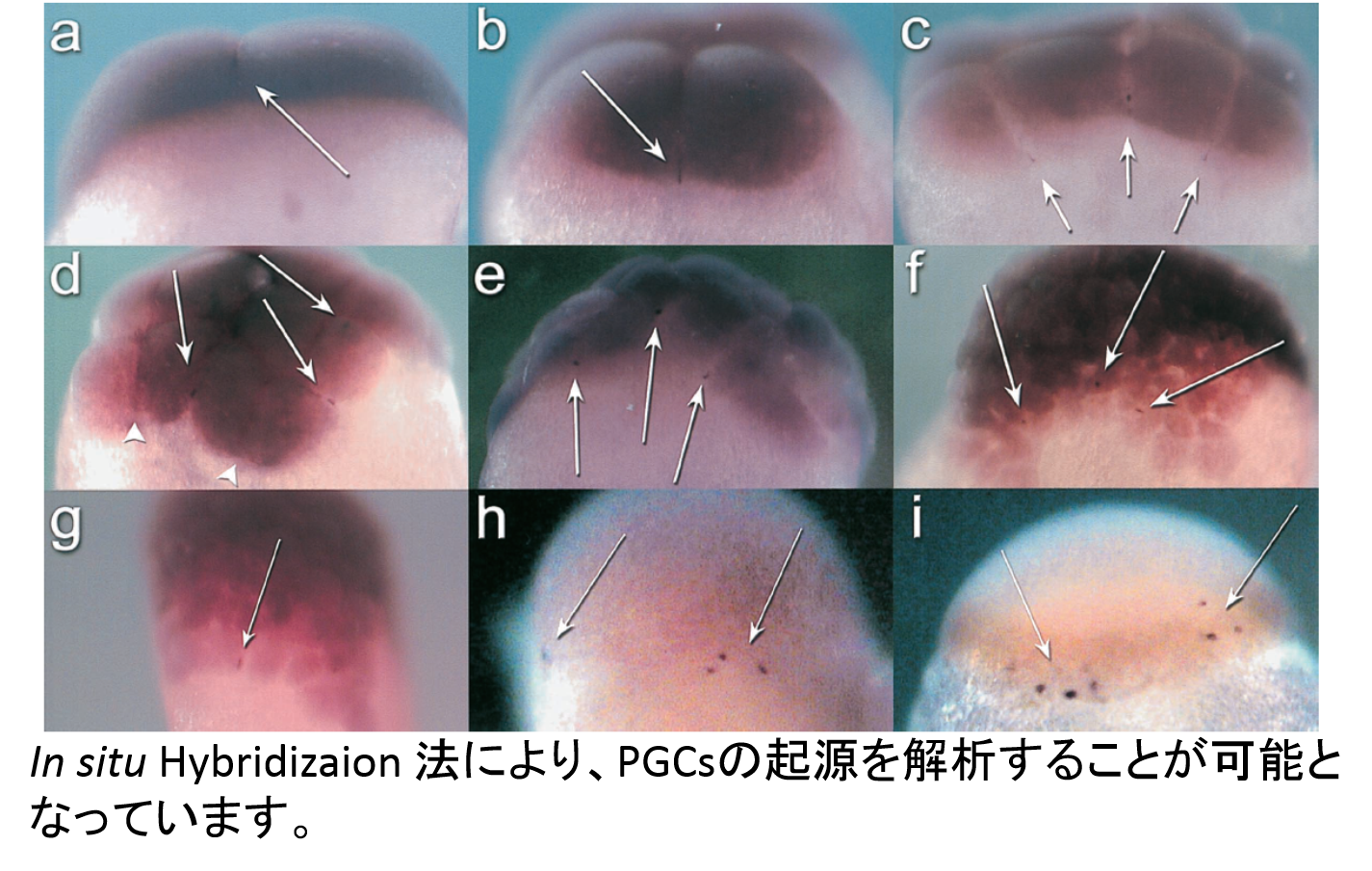
Analysis of biological
property of PGCs
As described above, all donor PGCs do not always move to gonadal area in
host embryos and differentiate in host gonad. The migration pathway
and differentiation of PGCs are not always clear in many teleost fish species
yet. Basic properties of PGCs in many kinds of teleost fish are required
for establishment of surrogate propagation techniques. Now, migration
pathway has been analysis in about 20 species, including barfin flounder,
loach, ice-goby, rainbow smelt, herring, eel and sturgeon.
Sterilization of host germ cells
In the germ-line chimeras, however, it is impossible to avoid differentiation of gametes derived from host PGCs; two types of gametes, one from the donor and the other from the host are obtained. This is not efficient for the surrogate production. Endogenous PGCs should be removed by artificial sterilization of the host. It has been reported that sterile fish are induced by hybrid crossing between two different species. For example, hybrid males between goldfish and common carp are completely sterile. Applying this technique, only sperms from the donor were obtained from the germ-line chimeras, in which goldfish PGCs were transplanted into the host blastula of the hybrid between goldfish and common carp. Inhibition of differentiation by morpholino treatment is also effective to inhibit host PGCs differentiation in surrogate production.
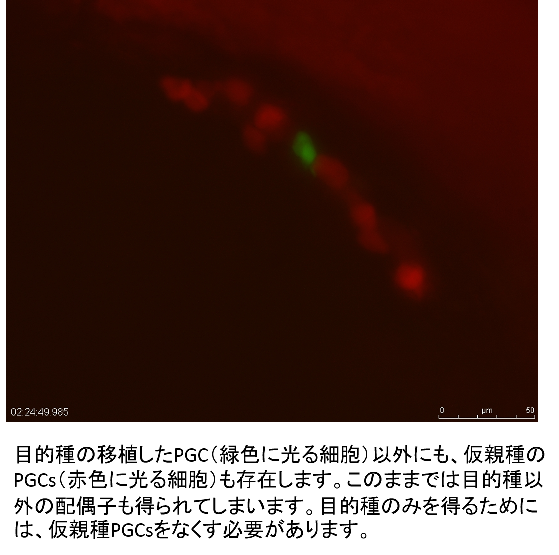
Fusion technique between
surrogate production and other bio-technology
In early stage of development, PGCs are only cell to differentiate gametes
thorough germ-line chimera. Modified changes in are confirmed phenotypically
in the next generation. Therefore, artificial modification in PGCs
will produce individuals with new properties. For example, tetraploid
PGCs may be induced by cell-fusion technology, resulting in diploid gametes.
These diploid gametes are useful for triploid and amphidiploid induction
by normal fertilization and that followed by inhibition of second maturation
division. Now, tetraploid PGCs have not induced yet, because it is
difficult to fuse a single pair of PGCs. A new idea is required.
Conclusion
In Japan, many teleost fish are used as food. The diversity in fish
used is wider than that in domestic animals. In the future, several
investigations are required in the following points; 1) improvement of
PGC isolation technique, 2) enhancement of PGC integration efficiency into
host genital anlage, 3) investigation of xenogeneic host which enabled
donor PGCs to differentiate, and 4) sterilization of host endogenous PGCs.
Manipulation apparatus and techniques
In this section, we introduce our techniques and apparatus for manipulation of fish embryos, using Nanae Fresh-Water Laboratory. In many case, goldfish is used as the material. Please check the references described in each section, too, if you want to know the detailed procedures.

Artificial fertilization
Embryos are prepared by artificial fertilization, because of synchronized
development and subsequent treatments, such as dechorionation. It
is important for cell and blastoderm transplantation to remove the chorion.
Dechorionation procedures are described below. Quality of eggs is
also important for subsequent development, but it depends on many factors,
such as cultivation of maternal fish and treatments from ovulation.
Eggs are striped onto the appropriated sheet or bowl by gentle pressing
from ovulated maternal fish. Sperms are collected into the capillary
tube in case of small fish, or to the small Falcon tubes in case of large
fish. It is recommended that collected sperm is diluted with artificial
seminal plasm and kept in the refrigerator or on ice before use.
Dilution prolongs the sperm motility. In goldfish, sperm collected
in the micro-capillary tube loses their activity for several hours, but
it keeps the activity for one week under diluted and lower temperature
conditions. Eggs are inseminated with sperm and fertilized by adding
appropriate medium for fertilization.
In the case of goldfish, eggs are collected onto the polyethylene or other
film and sperm is collected into the micro-capillary tube and diluted about
50-100 times with artificial seminal plasm (5.61 g NaCl, 5.23 g KCl, 0.33
g CaCl2・2H2O, 0.22 g MgCl2・6H2O, 0.2 g NaHCO3, diluted with 1 litter DDW).
Dechorionation
Chorion of fertilized eggs should be removed by appropriate treatment.
Eggs are inseminated with sperm and fertilized by adding appropriate medium
for fertilization. In the case of loach and zebrafish, eggs are fertilized
in tap water, while in goldfish, inseminated eggs are fertilized in tap
water containing 0.2% urea and 0.24% NaCl, because of removal of adhesive
materials around surface of chorion. Controls of pH condition or
physical treatment of fertilized eggs are occasionally required before
dechorionation in other teleost species. We can remove chorion of
fertilized eggs in cyprinid fish, a few smelt fish, salmonid fish with
thin chorion, European cat-fish and herring by proteolytic enzymes with
several modifications.
Dechorionation are performed by treatment of proteolytic enzymes, such
as trypsin, pancreatin, actinase and so on. In cyprinid species,
dechorionation is successively performed by treatment with trypsin solution,
containing small amount of urea in some case. In many case, the treatment
should be begun as soon after fertilization as possible. In goldfish,
it takes about 10 min within 4 hours after fertilization, but 2 or 3 hours
treatment are required in the embryos after 1 day.

Manipulation apparatus
Denuded eggs without chorion are fragile to manipulate. Therefore,
we handmake special apparatuses for manipulation, such as a dissecting
needle, transplant-needle, culture plate and so on.
Dissecting needle is shown in Figure. Thin part of Pasteur pipette
is melted by fire of fine gas burner and extended manually. Cut the
extended part at appropriate position and melt the tip of it to soften
the surface. For blastoderm transplantation, thinner glass wool is
attached to the tip of the dissecting needle with manicure, and cut it
leaving 1 to 2 mm long.
Agar-coated petri dishes are used for manipulation and cultivation of
dechorionated eggs, without injuring them. About 0.8 to 1 % of agar
is dissolved in Ringer’s solution at boiling temperature, and poured onto
the level petri-dish about 1 mm in thick. Glass petri dish with flat
surface is recommended, because agar is easily removed after experiment.
Dishes can be kept in refrigerator for weeks. The dish with
thick agar-coat is useful at photographing the spherical or motile embryo.
Embryos are able to be hold in the small slit or hole in agar-coat.
Chromosome set manipulation
Chromosome set manipulation is useful techniques for improvement of genetic
combination, induction of sterile fish and so on. There are two directions
of the treatments, namely reducing and increasing the genome size.
Haploidy is induced for reducing the genome size, and polyploidization
is performed for increasing the genome size. Haploid individuals
are induced by fertilization between normal gamete and genetically inactivated
gametes. Polyploid individuals are induced by fertilization followed
with the treatment of the second maturation division or the first cleavage.
Hydrostatic pressure is used for inhibition of the second maturation division
in salmonid and sturgeon fishes, and temperature treatments in cyprinid
fish.
Blastoderm transplantation
In cyprinid fish, especially goldfish, operation of
blastodisc is easily performed at the blastula stage. Blastodisc is cut by glass-wool attached to
needle, described above, in the agar coated petri-dish. During operation, embryo is settled on the
agar-plate filled with Ringer’s solution containing with 1.6% albumen. It is important for blastodisc operation to
add albumen to Ringer’s solution. In
plain Ringer’s solution without albumen, blastodisc scatters from the cut end
during operation, and it takes longer time to heal the wound around the
attached ends of transplanted part.
During
operation, embryo should be softly held by fine-forceps or something else not
to move. To prepare the graft,
glass-wool knife on the desired point of blastodisc is moved vertically down
toward the agar plate, and then, pushing to the surface of agar, is moved
slowly horizontally. Soft agar gel
(about 0.8%) is recommended in this operation.
At the same time, the enveloping layer of host blastodisc is
removed. Graft should be transplanted
onto the prepared cut end of other blastodisc within one min. As time go on, adhesion of the cut ends grew gradually
becomes difficult. The cut end does not attached to the surface
of blastodisc with enveloping layer.
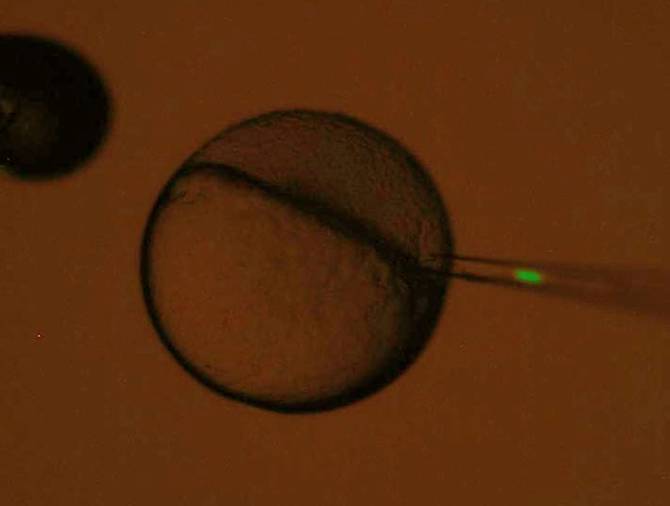
Single cell transplantation (SPT)
A single cell is picked up the injection needle, hold inside moved to
host embryo and inserted to the host embryo. Transplantation of single cell is very difficult, because picking up, holding
in the needle and transplantation of single depend on the many conditions,
such as needle (opening size of tip and constriction), injector, water
temperature, cell size and adhesion, mental condition and so on. It is
highly skillful.
We
use the needle with small constriction in hole size and small opening at the
tip. At first, petri dish filled without
agar coating and manipulator with micro-needle are installed under the
fluorescent stereo-microsocope. Dissociated
cells and host embryos are scattered in the petri dish. As the cells adhere to the matrix of petri
dish, some coating is required on the surface. The procedure of single cell transplantation
is quite complicated, and, therefore, described as the steps, as follows.
1) At higher level from the
bottom of the petri dish, move the tip of the micro-needle to the center of
focus with low magnification.
2) Move petri dish to the
position at the cells distributed.
3) Check the focus in the
cells with high magnification.
4) Move down the tip of the
needle to the cell level.
5) Move the tip to the
objective cells and suck into the needle with high magnification.
6) Move the tip to the host
embryo with low magnification, then transplanted into blastoderm.
Needles for microinjection and blastomere transplantation
We use four types of manipulation needles, such as 1) simple pulled needle,
2) needle with constriction, 3) needle which tip was sharpened with large
opening, and 4) needle with constriction and small opening. We make
all needles from Drammond micro-caps (50 ml) as material.
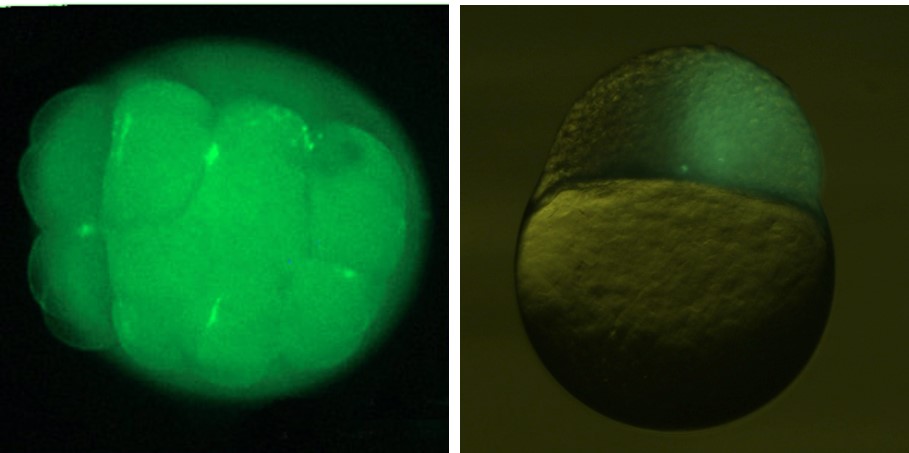
Blastomere transplantation (BT)
Blastomere transplantation at the blastula stage was firstly reported by
Lin et al. (1993) in zebrafish. We modified their procedures of transplantation.
In this operation, glass needle with wide opening at the tip and with constriction
is recommended. The inside of the glass needle is filled with Ringer’s
solution and also coated with chicken albumen or yolk of fish egg used,
not to adhere with blastomeres. During operation, donor and host
embryos are settled on the agar-plate filled with Ringer’s solution.
Holding the donor embryo with holding needle, the tip of transplantation
needle is inserted into the blastodisc. The donor blastomeres are
sucked and collected into the transplantation needle. When pulled
out the tip of the needle from donor blastodisc, collected blastomeres
moved slowly into inside and stopped in front of the constriction.
Then the needle is inserted into the host, and donor blastomeres are transplanted.
バナースペース
Nanae Fresh-Water Station
2-9-1, Sakuracho, Nanae town Kameda-gun, Hokkaido, 041-1105, Japan
TEL 0138-65-2344
FAX 0138-65-2239